Efficacy
Sofdra demonstrated significant sweat reduction1
Jump to:

—commercially insured patients pay $0*
Co-primary endpoints
Sweat reduction was evaluated in two ways in the phase 3 CARDIGAN trials1†
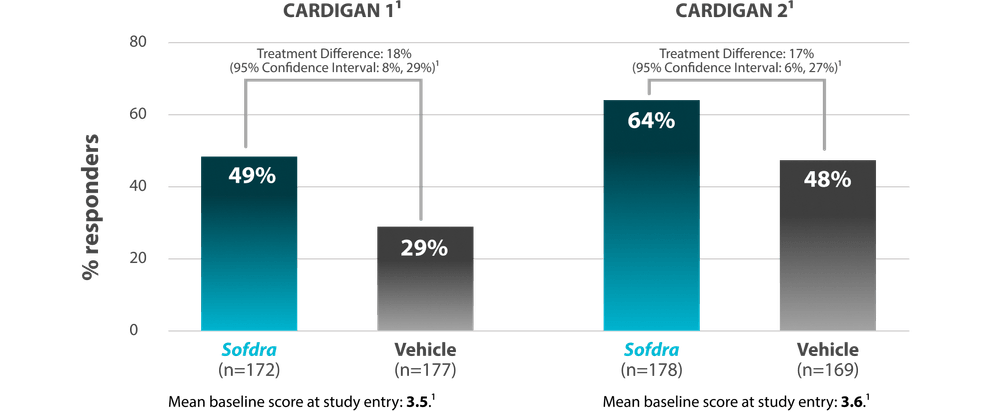
Patient-reported measure: ≥2-point improvement in HDSM-Ax-7 score from baseline to Day 43
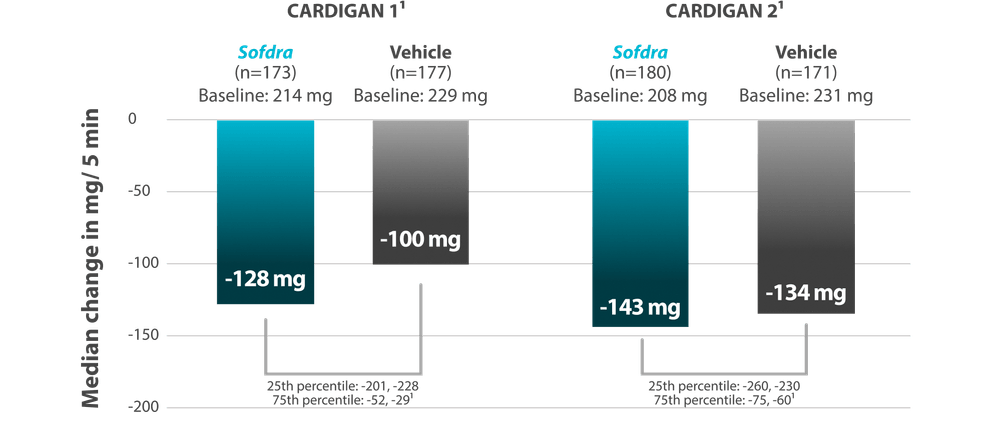
Objective measure: median reduction in GSP from baseline to Day 43
Pooled analysis of secondary endpoint
Sofdra demonstrated significant sweat reduction using a patient-reported measure2
of Sofdra patients
reported a ≥1-point change in
their HDSM-Ax-7 score at Day 43, a
clinically meaningful
improvement2
(n=319) (p=0.0002).
For patients using vehicle: 72.3% reported a ≥1-point improvement at Day 43 (n=325).2
Study design
Studied in two 6-week, double-blind, randomized, vehicle-controlled phase 3 trials of severe to very severe patients1
The studies included a broad population of 701 patients with primary axillary hyperhidrosis (Sofdra N=353; vehicle N=348)1:
- 20% Black or African American, 78% White
- 31% Hispanic or Latino
- 56% female, 44% male
- 10 to 65 years of age or older, with 91% between the ages of 18 and 64
- Mean baseline HDSM-Ax-7 score at study entry was 3.5 in CARDIGAN 1 and 3.6 in CARDIGAN 2
Efficacy assessments included:
Co-primary endpoints:
- ≥2-point improvement in HDSM-Ax-7 score from baseline to Day 43 (patient-reported measure)1
- Median reduction in GSP (mg/5 min) from baseline to Day 43 (objective measure)1
Key secondary endpoints:
- ≥1-point improvement in HDSM-Ax-7 score from baseline to Day 432
- Combined analyses of improvement in HDSM-Ax-7 score and GSP reduction2
The Hyperhidrosis Disease Severity Measure-Axillary-7 (HDSM-Ax-7)
The HDSM-Ax-7 is a validated measure of 7 frequently mentioned daily concerns of axillary hyperhidrosis patients, including, but not limited to sweating2:

When nervous

That causes damp or
wet clothing

When sedentary

In cooler temperatures
Questions used for HDSM-Ax-7 as a patient-reported outcome assessment2
Since you woke up yesterday, how severe was your experience with:
Gravimetric sweat production (GSP)

An objective measure of sweat production
- GSP is assessed by placing a piece of filter paper on the axilla for 5 minutes2
- The filter paper is weighed before and after application to measure sweat production in terms of weight over time2
in an open-label, long-term trial.1

Indication
Sofdra™ (sofpironium) topical gel, 12.45% is an anticholinergic indicated for the treatment of primary axillary hyperhidrosis in adults and pediatric patients 9 years of age and older.
IMPORTANT SAFETY INFORMATION
Contraindications
Sofdra is contraindicated in patients with medical conditions that can be exacerbated by the anticholinergic effect of Sofdra (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis, Sjögren’s syndrome).
Warnings & Precautions
Urinary retention
Use Sofdra with caution in patients with a history or presence of documented urinary retention. Patients should discontinue use immediately and consult a healthcare provider should any signs or symptoms of urinary retention develop (e.g., difficulty passing urine, distended bladder).
Control of Body Temperature
In the presence of high ambient temperature, heat illness (hyperpyrexia and heat stroke due to decreased sweating) can occur with the use of anticholinergic drugs, including Sofdra. Patients should avoid using Sofdra if not sweating when in hot or very warm environmental temperatures.
Operating Machinery or an Automobile
Transient blurred vision may occur with Sofdra. If blurred vision occurs, discontinue use and avoid engaging in activities that require clear vision (e.g., operating a motor vehicle or machinery, performing hazardous work) until the symptoms have resolved.
Adverse Reactions
The most common adverse reactions (≥2%) are dry mouth, vision blurred, mydriasis, and urinary retention. The most common local skin reactions (≥2%) are pain, erythema, dermatitis, pruritus, irritation, and exfoliation.
Drug Interactions
Avoid coadministration of Sofdra with other anticholinergic medications (due to risk of increase in anticholinergic adverse effects) and drugs that are strong inhibitors of CYP2D6.
Please see full Prescribing Information.